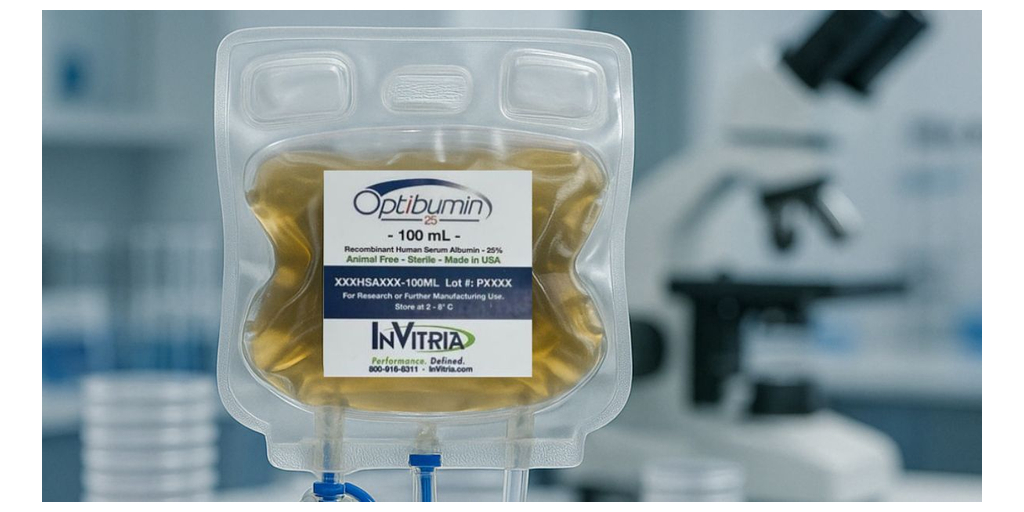
JUNCTION CITY, Kan.–(BUSINESS WIRE)–#Biomanufacturing–InVitria, a leading developer of recombinant, chemically defined components for biomanufacturing, has announced the launch of Optibumin® 25, the first and only recombinant human serum albumin (rHSA) available as a 25% solution. Designed for closed-system workflows, Optibumin 25 is a high-purity alternative to plasma-derived albumin, enabling safer, more consistent manufacturing in cell and gene therapy applications.
For decades, plasma-derived HSA has presented regulatory and supply chain challenges, including batch variability and pathogen risks. Optibumin 25 eliminates these issues by offering a chemically defined, animal-origin-free, GMP-compliant alternative. It supports regulatory compliance while improving scalability, sterility, and formulation performance.
“Optibumin 25 directly replaces plasma-derived 25% HSA while enhancing process control and reducing regulatory risk,” said Scott Deeter, CEO at InVitria. “It enables the kind of consistency required for clinical and commercial-scale production.”
Manufactured in an ISO 9001-certified, cGMP-compliant facility, Optibumin 25 is optimized for downstream processing, formulation, and cryopreservation workflows. The product is currently available in 100 mL single-use bags and is suitable for integration into viral vector manufacturing, stem cell preservation, vaccine formulation, and regenerative medicine.
Key Benefits:
- First recombinant 25% HSA designed for closed systems
- Animal-origin-free and chemically defined
- GMP-ready and scalable to commercial volumes
- Stable, sterile, and contamination-resistant
To learn more visit:
www.invitria.com/optibumin25
About InVitria
InVitria develops and manufactures high-performance, animal-origin-free supplements and excipients that support regulatory compliance and product consistency in biologics manufacturing. Its recombinant portfolio enables safer, scalable alternatives to blood-derived materials in cell and gene therapy, vaccine development, and regenerative medicine. InVitria products are manufactured in the United States in cGMP-compliant, ISO 9001-certified facilities.
Contacts
Alexia Armstrong
Director of Marketing
[email protected]
InVitria, Inc.
2718 Industrial Drive, Junction City, KS 66441
Phone: 401-636-1969

.jpg)
.jpg)
