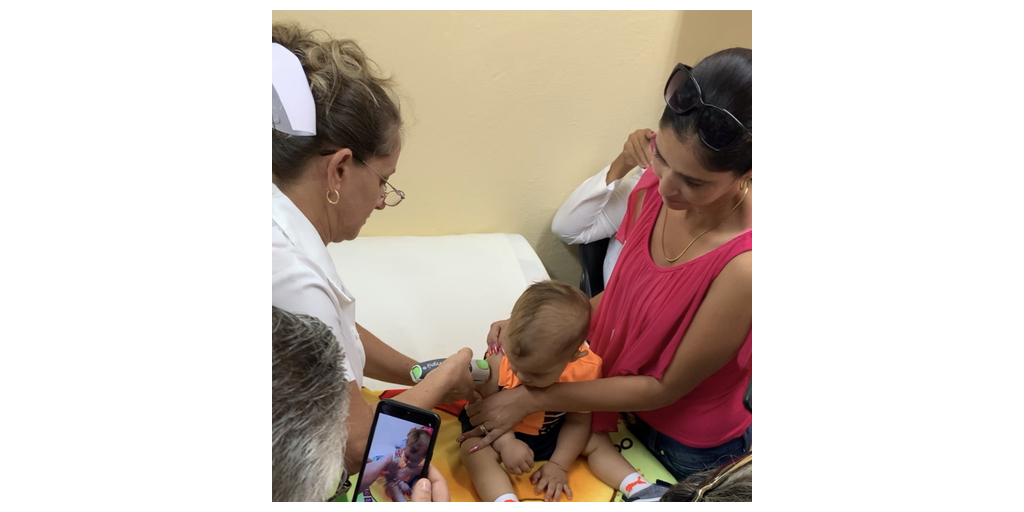
- There were significantly fewer adverse events associated with needle-free Tropis® intradermal (ID) delivery as compared to needle and syringe (N/S).
- Tropis ID delivery was preferred over N/S ID delivery by more than 98% of parents and healthcare staff.
- Seroprevalence levels were comparable between the two methods of administration.
GOLDEN, Colo.–(BUSINESS WIRE)–PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its needle-free injection technology, today announced the publication of a study demonstrating the benefits of the PharmaJet Tropis needle-free system compared with N/S for ID administration of fractional dose poliovirus vaccine (fIPV). The study entitled Tropis needle-free injector for fractional-dose IPV administration: A pilot study for integration into routine immunization services in Cuba is published in Vaccine.1
In Cuba, the routine immunization (RI) schedule for polio consists of two doses of fIPV administered at 4 and 8 months of age administered intradermally with N/S. However, ID administration with N/S is relatively uncomfortable, complex and requires specific training. Improper technique can result in reduced immunogenicity.2 Tropis offers an easy to use, reliable technique that has been shown to improve coverage in Pakistan,3 Somalia4 and Nigeria,5 where it has been used to vaccinate nearly 12 million children. Tropis is the first and only needle-free ID delivery technology to achieve World Health Organization (WHO) prequalification.
The study measured acceptability, safety, and immunogenicity of Tropis compared to N/S administration with a total of 6,704 children vaccinated. Surveys were administered to parents/guardians (n=5260) and nurses (n=66) to evaluate satisfaction. Additionally, blood samples from vaccinated children were analyzed for neutralizing poliovirus antibody levels.
The results indicated significantly fewer adverse events with Tropis (6%) than N/S (13%) (p=0.028), and Tropis was preferred over N/S by large margins by both parents (98%) and nurses (98.5%). Survey respondents cited ease of vaccination, diminished crying, and increased comfort as the benefits of Tropis. Of note, seroprevalence did not differ significantly between Tropis and N/S.
This research collaboration between the Instituto Pedro Kourí (IPK) and the WHO was conducted to gain valuable insights that can be applied to curb the global circulating vaccine-derived poliovirus. Cuba has proven a useful resource and partner for polio eradication, acting as an incubator for testing new ideas and approaches. The WHO–Cuban collaboration, lasting for over 20 years, has been vital for the Global Polio Eradication Initiative (GPEI), making it possible to design innovative strategies, especially for the polio eradication endgame and for immunization policy development worldwide.6
“These results highlight that Tropis results in fewer AEs than Mantoux technique and is more acceptable. This should be very encouraging to vaccine programs aiming to take advantage of ID delivery,” said Paul LaBarre, Vice President of Global Business Development, PharmaJet. “The strong performance of Tropis ID delivery is consistent with results recently seen in WHO-sponsored polio campaigns in Pakistan, Nigeria, and Somalia where Tropis has been used to administer polio vaccinations to nearly 12 million children.”
For more information visit the PharmaJet website https://pharmajet.com.
Refer to Instructions for Use to ensure safe injections and to review risks.
About PharmaJet
The PharmaJet mission is to improve the performance and outcomes of injectables with our enabling technology that better activates the immune system. We are committed to helping our partners realize their research and commercialization goals while making an impact on public health. PharmaJet Precision Delivery Systems™ can improve vaccine effectiveness, allow for a preferred patient and caregiver experience, and offer a proven path to commercialization. They are also safe, fast, and easy-to-use. The Stratis® System has U.S. FDA 510(k) marketing clearance, CE Mark, and WHO PQS certification to deliver medications and vaccines either intramuscularly or subcutaneously. The Tropis® System has CE Mark and WHO PQS certification for intradermal injections. They are both commercially available for global immunization programs. For more information or if you are interested in partnering with PharmaJet visit https://www.pharmajet.com or contact PharmaJet here. Follow us on LinkedIn.
About Cuba’s role in the collaboration with the WHO and GPEI2
Cuba’s part in the continuing collaboration with the World Health Organization (WHO) has focused on issues relevant to policymaking for the ongoing global polio eradication effort. It has concentrated particularly on looking for answers to scientific questions that could not be answered elsewhere, taking into account Cuba’s unique OPV vaccination program, conducted only twice annually, usually in February and April. Specifically, research has addressed several aspects of OPV and inactivated poliovirus vaccine (IPV), such as immunogenicity, adverse reactions and complications, persistence of Sabin virus in populations, the immunogenicity schedule and its affordability, number of doses needed, evaluation of new vaccines and devices, and booster response.
Contacts
Nancy Lillie
[email protected]
1-888-900-4321 Option 3